Abstract
BACKGROUND: The median age for diagnosis of acute myeloid leukemia (AML) is 68 years. Elderly patients are often ineligible for intensive chemotherapy and have limited treatment options. Venetoclax (Ven), an oral agent that targets the antiapoptotic protein, BCL-2, has demonstrated high rates of remission (>60%) when administered in combination with low-dose cytarabine (LDAC), and could represent a potent therapeutic option for patients ineligible for intensive chemotherapy.
METHODS: This open-label, phase 1/2 study (NCT02287233) evaluated the safety and efficacy of venetoclax in combination with LDAC in patients with previously untreated AML who were ineligible for intensive chemotherapy due to comorbidities or age. Patients had an Eastern Cooperative Oncology Group (ECOG) performance score of 0-2, had adequate hepatic and renal function, and were enrolled from December 2014 to May 2017. In the dose escalation portion of the study, 600 mg venetoclax was determined to be the recommended phase 2 dose (RPTD). Venetoclax was initiated at 50 or 100 mg daily and dose escalated over 4-5 days to reach the RPTD. In subsequent 28 day cycles, venetoclax was administered at 600 mg on all days. LDAC (20 mg/m2 daily) was subcutaneously administered on days 1-10 of each cycle. At the beginning of the study, concominant strong and moderate CYP3A inhibitor use was prohibited; however, as additional safety and pharmacokinetic data became available, their use was allowed with appropriate venetoclax dose adjustments. Time to first response, rates of complete remission (CR), CR with incomplete blood count recovery (CRi), CR with partial hematologic recovery (CRh), duration of response, achievement of transfusion independence, overall survival (OS) and adverse events (AEs) were evaluated. Minimal residual disease (MRD) was evaluated centrally by multicolor flow cytometry at a cutoff of 10-3 leukemic cells.
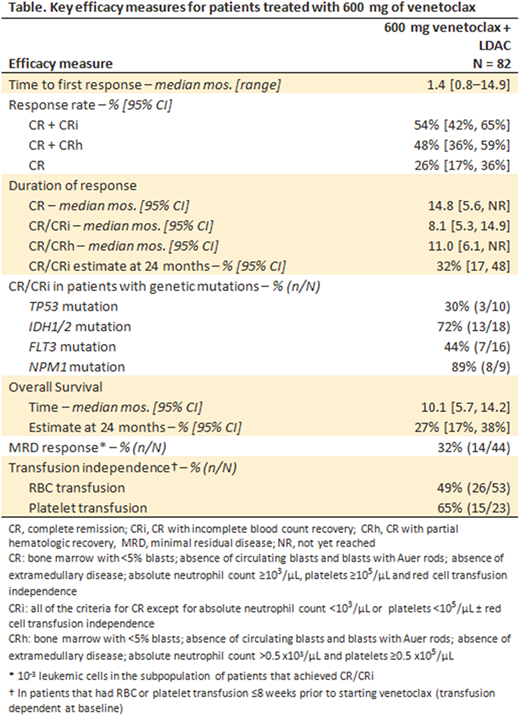
RESULTS: Data cutoff was November 8, 2017. Of 82 patients treated with 600 mg of venetoclax, 65% were male, 95% white race, 60% had intermediate and 32% had poor cytogenetic risk, and 49% had secondary AML (of whom 60% had prior HMA exposure). Transfusion dependence for red blood cells (RBC) and platelets within 8 weeks prior to venetoclax treatment was 65% (53/82) and 28% (23/82) of patients, respectively. Most common grade ≥3 AEs across all patients were febrile neutropenia (43%), thrombocytopenia (38%), neutropenia (27%), and anemia (27%). Laboratory evidence of grade 3 tumor-lysis syndrome (TLS) was observed in two patients; both patients achieved the target dose of venetoclax. Forty seven percent of patients received moderate (40%) or strong (7%) CYP3A inhibitors for at least 7 days (predominantly azole antifungals); no relevant differences in serious adverse event rates were observed. Key efficacy results are shown in the Table. Median time to first response was 1.4 months, and 54% and 46% of patients achieved CR/CRi and CR/CRh, respectively. The rates of CR/CRi for patients with secondary and de novo AML were 35% and 71%, respectively; median DOR for those with secondary and de novo AML was 8.1 and 11.6 months, respectively. Patients with selected genetic mutations achieved the following rates of CR/CRi: TP53, 30%; IDH1/2, 72%; FLT3, 44%; NPM1, 89%. MRD response below 10-3 cutoff was achieved by 32% of patients with CR/CRi; median OS has not yet been reached for these patients. Among patients that were RBC or platelet transfusion dependent at baseline, 49% (26/53) and 65% (15/23), respectively, achieved transfusion independence while on venetoclax therapy.
CONCLUSIONS: Venetoclax in combination with LDAC led to rapid, deep, and durable responses in patients with AML who were ineligible for intensive chemotherapy. Venetoclax plus LDAC demonstrated an improved CR rate (26% vs. 8%), CR/CRi rate (54% vs. 11%) and median overall survival (10 months vs. 5 months) compared to the historical rates with LDAC alone. Furthermore, a majority of patients achieved transfusion independence during venetoclax therapy. Strong and moderate CYP3A inhibitors, including azole antifungals, were safely coadministered with appropriate venetoclax dose adjustments. These results demonstrate that venetoclax, in combination with LDAC, represents an effective therapeutic option for patients with AML who are not suitable for standard induction therapy.
Wei:Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Strickland:Boehringer Ingelheim: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma: Consultancy; Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tolero Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunesis Pharmaceuticals: Consultancy, Research Funding. Fiedler:Amgen: Consultancy, Other: Meeting attendance, Patents & Royalties, Research Funding; ARIAD/Incyte: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Gilead: Other: Meeting attendance; GSO: Other: Meeting attendance; Teva: Other: Meeting attendance; JAZZ pharma: Other: Meeting attendance; Daiichi Sankyo: Other: Meeting attendance. Lin:Jazz Pharmaceuticals: Honoraria. Walter:Actinium Pharmaceuticals, Inc: Other: Clinical Trial support , Research Funding; Amgen Inc: Other: Clinical Trial Support, Research Funding; Amphivena Therapeutics, Inc: Consultancy, Other: Clinical Trial Support, Research Funding; Aptevo Therapeutics, Inc: Consultancy, Other: Clinical Trial Support, Research Funding; Covagen AG: Consultancy, Other: Clinical Trial Support, Research Funding; Seattle Genetics, Inc: Consultancy, Other: Clinical Trial Support, Research Funding; Pfizer, Inc: Consultancy; Boehringer Ingelheim Pharma GmbH & Co. KG: Consultancy. Hong:Genentech Inc/Roche: Employment, Other: Ownership interests PLC. Chyla:AbbVie, Inc: Employment, Equity Ownership. Popovic:AbbVie Inc: Employment, Equity Ownership. Fakouhi:AbbVie, Inc: Employment, Equity Ownership. Xu:AbbVie, Inc: Employment, Equity Ownership. Hayslip:AbbVie: Employment, Equity Ownership. Roboz:Bayer: Consultancy; Pfizer: Consultancy; Bayer: Consultancy; AbbVie: Consultancy; AbbVie: Consultancy; Orsenix: Consultancy; Cellectis: Research Funding; Aphivena Therapeutics: Consultancy; Otsuka: Consultancy; Roche/Genentech: Consultancy; Astex Pharmaceuticals: Consultancy; Orsenix: Consultancy; Roche/Genentech: Consultancy; Daiichi Sankyo: Consultancy; Jazz Pharmaceuticals: Consultancy; Eisai: Consultancy; Celgene Corporation: Consultancy; Sandoz: Consultancy; Novartis: Consultancy; Celgene Corporation: Consultancy; Cellectis: Research Funding; Argenx: Consultancy; Jazz Pharmaceuticals: Consultancy; Daiichi Sankyo: Consultancy; Sandoz: Consultancy; Janssen Pharmaceuticals: Consultancy; Astex Pharmaceuticals: Consultancy; Janssen Pharmaceuticals: Consultancy; Celltrion: Consultancy; Aphivena Therapeutics: Consultancy; Argenx: Consultancy; Celltrion: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Otsuka: Consultancy; Eisai: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal